Fluoroquinolone Acquired Disability
Fluoroquinolone-Associated Disability (FQAD) is a delayed, multisystem condition caused by fluoroquinolone antibiotics.
It reflects drug-induced mitochondrial dysfunction (DIMD)—a form of cellular energy injury that can continue and worsen after the drug is stopped.
This is not a collection of temporary side effects, but a predictable biological process that affects multiple organ systems.
What Is FQAD?
Fluoroquinolone-Associated Disability (FQAD) is a severe, often delayed condition that can follow exposure to fluoroquinolone antibiotics. It is one of the most clearly characterized forms of drug-induced mitochondrial dysfunction (DIMD), in which cellular energy production and repair capacity are progressively impaired rather than acutely destroyed.
Fluoroquinolone Antibiotics
Fluoroquinolones are widely prescribed (even with SEVEN BLACK BOX WARNINGS) for routine infections and long considered safe for short-term use. Common agents include:
- Ciprofloxacin
- Levofloxacin
- Moxifloxacin
- Ofloxacin
- Norfloxacin
These medications were frequently prescribed in outpatient settings and often used repeatedly, without recognition of cumulative cellular injury.
Why Fluoroquinolones Are Different
Fluoroquinolones directly impair mitochondrial DNA and mitochondrial enzymes, disrupt oxidative phosphorylation, induce oxidative stress, and interfere with collagen synthesis and connective tissue repair. Together, these effects compromise the cell’s ability to produce energy and complete normal repair processes.
Importantly, injury does not necessarily stop when the medication is discontinued. Mitochondrial damage can persist and progress after exposure ends, leading to delayed and cumulative energy failure over time. This progressive pattern is the feature most often missed in conventional clinical models.
Fluoroquinolones impair mitochondrial energy production through direct disruption of the electron transport chain and mitochondrial DNA. The mechanisms below illustrate how fluoroquinolones disrupt mitochondrial energy production at the electron transport chain level.
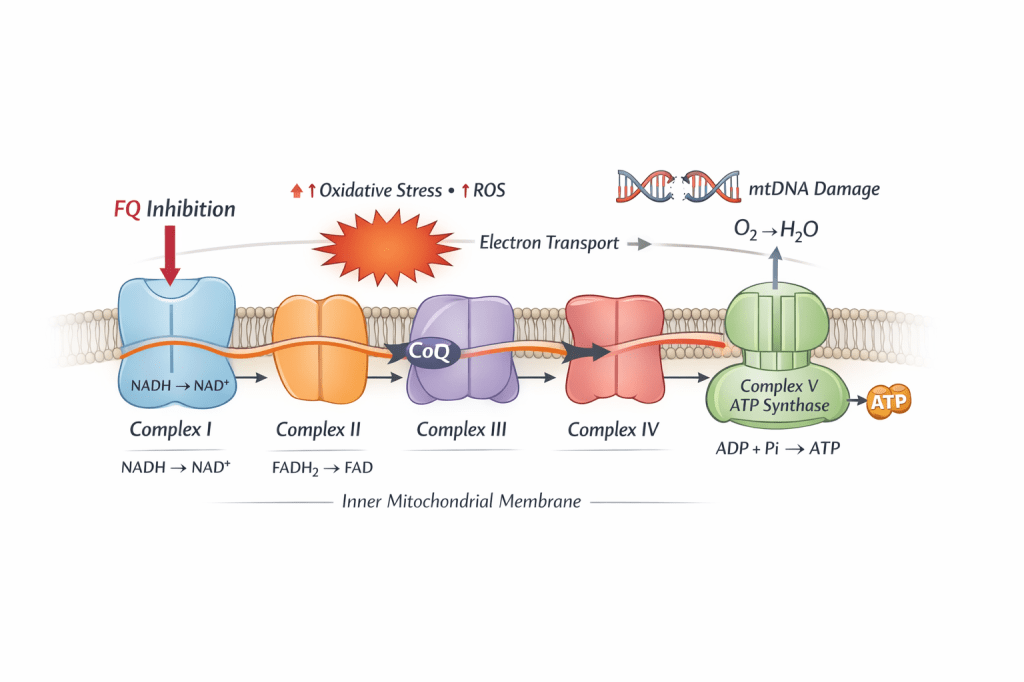
Mitochondrial electron transport chain highlighting fluoroquinolone-associated Complex I inhibition. Disruption at Complex I increases oxidative stress and reactive oxygen species (ROS), contributes to mitochondrial DNA injury, and impairs ATP production across the energy system.
A Multisystem Pattern of Injury
Because mitochondria function as the shared energy infrastructure of the body, fluoroquinolone injury does not present as a single-organ disease. Instead, it produces a recognizable multisystem pattern affecting high-energy tissues.
Patients may experience neurologic and cognitive symptoms (such as neuropathy, brain fog, and dysautonomia); musculoskeletal and connective tissue injury (including tendon damage and joint instability); autonomic and cardiac abnormalities (heart rate or blood pressure instability); endocrine and metabolic dysfunction (severe fatigue and stress intolerance); and neuropsychiatric symptoms (anxiety, panic, and emotional lability). In this context, these symptoms reflect impaired cellular energy metabolism rather than primary psychological disease.
Why This Is Not “Just Side Effects”
Fluoroquinolones share a DNA-damaging, topoisomerase-poisoning mechanism with certain chemotherapies, but the clinical outcomes differ because of how these drugs are used. Chemotherapy is given at high doses for a defined period, with toxicity expected, monitored, and acknowledged.
Fluoroquinolones are prescribed at lower doses for routine infections and are often given repeatedly. Their mitochondrial effects are rarely monitored or recognized as cytotoxic. As a result, damage to mitochondrial DNA and energy production can accumulate silently rather than causing immediate cell death. Cancer cells may die. Bacteria may die. Mitochondria usually persist—damaged, unstable, and progressively dysfunctional. This distinction explains the delayed, multisystem nature of FQAD.
People have a right to know this process is occurring in their bodies. These outcomes are not random side effects or idiosyncratic reactions; they reflect a predictable biological mechanism of injury. Unlike transient side effects, mitochondrial injury can persist and worsen over time. Accurate language matters, because meaningful informed consent is not possible when mechanisms of injury are minimized or mischaracterized.
Regulatory Recognition Gaps
Despite accumulating evidence of mitochondrial toxicity, regulatory warnings historically focused on isolated organ-specific risks rather than a unifying energy-system injury. As a result, patients were often repeatedly re-exposed to medications that further impaired mitochondrial function, without screening, longitudinal monitoring, or informed discussion of cumulative risk.
FQAD as a Model for DIMD
FQAD demonstrates how drug-induced mitochondrial injury can present as a delayed, progressive, multisystem condition—a pattern now recognized across many other medications beyond fluoroquinolones. In this way, FQAD serves as a model condition, not an anomaly.
Recognition Matters
Many patients with FQAD were told their symptoms were unrelated, rare, or psychological. We now understand these outcomes reflect predictable consequences of mitochondrial injury—not coincidence, not weakness, and not isolated reactions. Recognition is the first step toward prevention, appropriate care, and informed consent.
Scientific basis: The mechanisms described on this page are supported by peer-reviewed literature on fluoroquinolone-induced mitochondrial dysfunction, topoisomerase poisoning, oxidative stress, and delayed toxicity.
See the Evidence & References page for source material.